Respuesta :
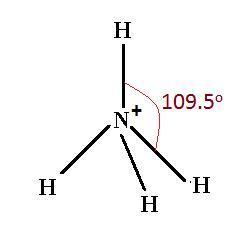
Answer is: 109,5°.
Nitrogen has eight electrons in the outer level. There are therefore four pairs, all of which are bonding because of the four hydrogens.
The ammonium ion has exactly the same shape as methane, because it has exactly the same electronic arrangement, ammonium ion is tetrahedral (sp3 hybridized.
Nitrogen has eight electrons in the outer level. There are therefore four pairs, all of which are bonding because of the four hydrogens.
The ammonium ion has exactly the same shape as methane, because it has exactly the same electronic arrangement, ammonium ion is tetrahedral (sp3 hybridized.
Answer : The angle between two of the nitrogen-hydrogen bonds in the ammonium ion [tex](NH_4^+)[/tex] is, [tex]109.5^o[/tex]
Explanation :
Formula used :
[tex]\text{Number of electrons}=\frac{1}{2}[V+N-C+A][/tex]
where,
V = number of valence electrons present in central atom
N = number of monovalent atoms bonded to central atom
C = charge of cation
A = charge of anion
First we have to determine the hybridization of the [tex]NH_4^+[/tex] molecules.
[tex]\text{Number of electrons}=\frac{1}{2}\times [5+4-1]=4[/tex]
The number of electrons are 4 that means the hybridization will be [tex]sp^3[/tex] and the electronic geometry of the molecule will be tetrahedral and the bond angle will be, [tex]109.5^o[/tex].
Hence, the angle between two of the nitrogen-hydrogen bonds in the ammonium ion [tex](NH_4^+)[/tex] is, [tex]109.5^o[/tex]